Oppsummering av matverksted 11/2019
Vi er nok flere som ikke har tenkt alt for hardt på alle de ulike mulighetene vi har når/hvis vi skal lage marengs. Det finnes flere ulike typer marengs som brukes til ulike ting: klassisk (fransk), italiensk, sveitisisk. Og de seinere årene har vi lært at vi ikke en gang trenger egg når vi skal laget det; bare åpne en pakke med kikerter, sil av væsken og pisk i vei. Men smaker det likt? Gir det samme konsistens? Må den varmes opp mindre, like mye, eller kanskje mer? Vi testet ut dette vårt åpne matverksted hos Klippfiskakademiet i Ålesund 11. november.Marengs - En enkel (og ganske ufullstendig) oversikt
Fransk marengsMetode: Bland eggehviter og sukker, pisk, stek i ovn.
Egenskaper: Blir sprø, kan være myk inni
Bruksområde: pikekyss, pavlova osv.
Italiensk marengs
Metode: Varm opp sukkerlake (til f.eks. 119 °C). Pisk eggehviter, hell sukkerlaken i en tynn stråle mens du pisker.
Egenskaper: Blir seig og relativt "kremete"
Bruksområde: F.eks. topping på pai som deretter brunes med gassbrenner
Sveitsisk marengs
Metode: Bland eggehviter og sukker, sett bollen på damp-/vannbad og rør/pisk til blandingen holder en viss temperatur. Ta til side og pisk stiv (finnes dog i flere ulike varianter)
Egenskaper: Blir seig og relativt "kremete"
Bruksområde: Vanlig som base der man etterpå pisker inn mykt smør/margarin for å få krem/topping til cupcakes ol.
Aquafaba-marengs
Marengs der eggehvite er erstattet av vannet/væsken fra f.eks. kikerter. Vegansk alternativ til eggehvite. Aquafaba = vannet fra hermetiske kikerter eller andre belgfrukter (fra latin: aqua = vann, faba = bønner).
Problem/spørsmål
Ulike marengsoppskrifter gir ganske ulike fremgangsmåter, særlig for den sveitsiske typen. Hvor mye må man varme? Noen sier 85 °C, andre ber om langt lavere temperaturer. Noen steder oppgis ikke temperatur i det hele tatt. Hva er rett? Egg vil jo bli kokt ved så høy temperatur som 85 °C, vil det ikke? Og gjelder samme regler når vi skal lage marengsen av kikertvann, eller er det helt annerledes?Litt bakgrunnskunnskap
Når vi pisker luft inn i eggehviter eller kikertvann lager vi et skum, akkurat som når vi pisker krem av fløte. Begge to er et væskeskum/flytende skum: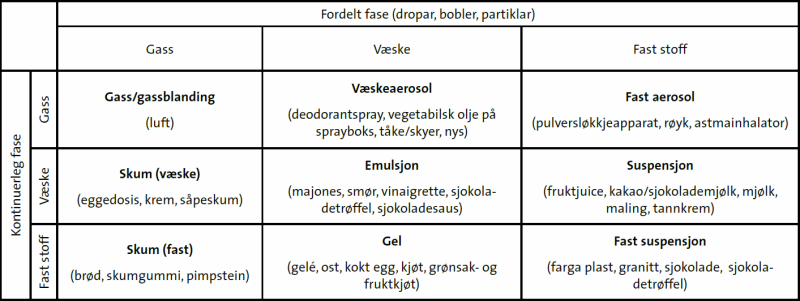 |
| Diagram fra artikkelen Sjokolade er ein dispersjon på naturfag.no/mat |
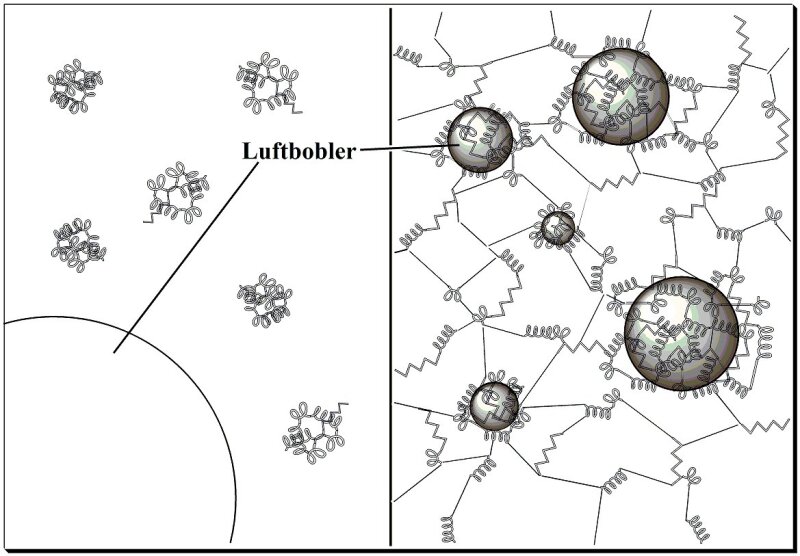 |
| Et eggehviteskum blir til. Fra artikkelen Å fange luft med egg på naturfag.no/mat |
Sukkeret gjør at vannet som omgir boblene blir mer tyktflytende (visøkst), noe som også hjelper til med å holde skummet stabilt fordi vannet ikke renner så lett av boblene.
Hva så med aquafaba, kikertvannet? Jeg har ikke konkret informasjon om innholdet i selve kikertvannet, da næringsstoffoversikten på kikertpakkene naturlig nok også omfatter kikertene og ikke bare kokevæsken. Det finnes noe forskning på området, men dette er ganske ferske saker og det er f.eks. lite å finne i fagbøker; det meste må man grave fram fra forskningsartikler eller mindre formelle nettsteder. Siden belgfrukter inneholder både stivelse og proteiner kan begge disse spille en rolle i å stabilisere skummet. Hvis stivelsen er viktig burde vi vente at det krevdes mer oppvarming for å få hjelp fra denne, akkurat som når man koker saus med potetmel, maizena eller hvetemel som jevning. Proteiner er så mangfoldige at her er mye åpent. Bordet var således dekket for et dobbelt sammenligningsforsøk: både mellom egg og kikerter, og mellom ulike temperaturer.
Forsøksoppsett
Vi laget fire parallelle sveitsiske marengser:- Eggehvite, varmet til 60 °C
- Eggehvite, varmet til 80 °C
- Kikertvann, varmet til 80 °C
- Kikertvann, kokende
| Variant med eggehvite | Variant med kikertvann |
| 3 eggehviter | 200 g silt kikertvann |
| 180 g sukker | 200 g sukker |
| 2 krm hvit eddik | 2 krm hvit eddik |
I de tre første parallellene rørte vi sammen sukker og eggehvite/kikertvann, varmet det opp over kokende vannbad til ønsket temperatur (damp, ikke direkte kontakt med vannet). Trekk til side og pisk til den ikke blir stivere, maks 10 minutter. I fjerde parallell ble kikertvannet kokt til det fikk en noe tykkere konsistens, sukker tilsatt og satt kjølig. Deretter tatt ut og pisket til stiv, inntil 15 minutter.
Nærmest for moro skyld prøvde vi også å steke topper av de fire i ovnen, og vi testet hvordan de fungerte når de ble brunet under gassflamme. Det skulle vi ikke angre på.
Nærmest for moro skyld prøvde vi også å steke topper av de fire i ovnen, og vi testet hvordan de fungerte når de ble brunet under gassflamme. Det skulle vi ikke angre på.
Resultater
Denne gangen fikk vi svært så klare resultater, nesten overraskende tydelige. Ofte er slike blindsmakinger vanskelige, og stemmene fordeler seg gjerne på de ulike parallellene. Denne gangen var det flere tilfeller der samme prøve fikk alle stemmene. |
| Resultat fra blindsmaking av de fire parallellene av sveitsisk marengs |
Utseeende til de fire etter ulike behandlinger var også ganske opplysende:
 |
| Før steking/bruning |
 |
| Etter bruning med gassbrenner (en anelse for mye; forfatteren tar det fulle ansvar) |
 |
| Etter steking i ovn |
Vi samlet også inn beskrivende ord for de fire:
Oppsummering
Det synes tydelig at temperaturen spiller en stor rolle, kanskje mer enn råvaren, når det gjelder konsistens på skummet. Dessverre hadde vi en type kikerter med ganske mye tilsatt salt (0,8%), så dette bidro til ganske mye saltsmak. Men selv uten salt var det tydelig at kikertsmaken stakk igjennom, i hvert fall når marengsen ble smakt alene. Vi har tidligere testet fransk marengs av aquafaba/kikertvann som deretter har blitt stekt, og da er kikertsmaken svak, nesten umerkelig. Og skal marengsen tilsettes andre smakssterke ingredienser vil de kanskje overdøve ertesmaken? Når panelet fikk velge den de foretrakk trakk eggehvitene helt klart det lengste strået, men kanskje er det likevel potensial dersom man leker seg litt med ingredienser, temperaturer og framgangsmåte?
Våre finske venner med matforsker Anu Hopia og kokk/kokkelærer Tatu Lehtovaara i spissen gjorde nesten samme forsøk samme kveld, bare med litt lavere temperaturer på eggehvitene. De kom faktisk til ganske lignende resultater i den forstand at temperaturen syntes å ha kanskje enda større effekt enn råvaren. I begge tilfeller fikk eggehviteskum varmet til 60-65 °C flest stemmer for mest foretrukket, men det er kanskje like mye et spørsmål om hva man skal bruke skummet til?


















