So, my thought was: Since it's the temperature that counts rather than the time, I can keep the temperature at 64-65 °C, and the egg will be perfect (to my taste) no matter how long they are cooked: a fool proof method to eggs with tender solid white and soft yolk! The pictures show eggs cooked ad 65 and 68 °C for 6 and 26.5 hours, respectively.


Hervé This also writes about this: "[...]at 62 °C one of the proteins in the white (ovotransferrin) is cooked, but the yolk remains liquid because the proteins that coagulate first in this part of the egg require a temperature of 68 °C. Obviously this would mean longer cooking times, but the result is a perfectly cooked egg" (This, pp. 31)
The proof is in the pudding, so I tried cooking an egg at 65 °C exactly for an hour or more, and was to put it mildly surprised. The egg came out with a runny white but firm yolk!! These come out the same whether they're kept for half an hour (to ensure the same temperature throughout the egg) or 26
There may be several reasons for this, but one is found in McGee (pp 85): "[…]the major [egg white] protein, ovalbumin, doesn't coagulate until about 80 °C".
So, it seems, this time chemistry played me a trick. We will still have to rely on physics: the reason that we can have eggs with firm white and soft yolk is, when using a temperature well above the coagulation temperature, that the white is "overheated" before the temperature of the yolk passes the point where the yolk stiffens.
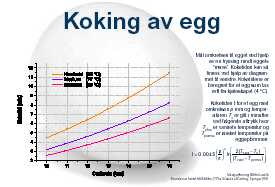
There is, by the way, a mathematic model/equation for this. Have a look at Martin Lersch's web site on Kitchen chemistry for a nice diagram and explanation of this.
Happy cooking
Erik
References and links:
Harold McGee: On Food and Cooking
Hervé This: Molecular gastronomy - Exploring the science of flavor
Martin Lersch's page "Molecular gastronomy and the science of cooking"
Post-comment (feb. 2009):
Douglas Balwin's excellent "A Practical Guide to Sous Vide Cooking" has a section on sous vide cooking eggs